Abstract
Sickle cell disease (SCD) is a group of autosomal recessive inherited disorders that affect approximately 100,000 people in the United States and millions worldwide. Liver or biliary complications including cirrhosis and cholestatic injury are evident in about 40% of hospitalized SCD patients. However, the molecular mechanisms responsible for sickle cell hepatopathy (SCH) remain poorly understood. Recently published studies and our preliminary data demonstrated impaired bile secretion and altered bile acid homeostasis accompanied red blood cell sequestration and fibrosis in the livers of humanized sickle cell mice. Although promising, a limitation of this model for the study of SCH is the predominance of hydrophilic bile acids in mice, which contrasts with a more hydrophobic and injurious bile acid pool in humans. A recent breakthrough in this area was the identification of murine-specific cytochrome P450 (Cyp2c70) as the enzyme responsible for the synthesis of mouse hydrophilic primary bile acids. Therefore, we hypothesize that the human hydrophobic bile acid composition would promote the development of cholestatic sickle cell disease.
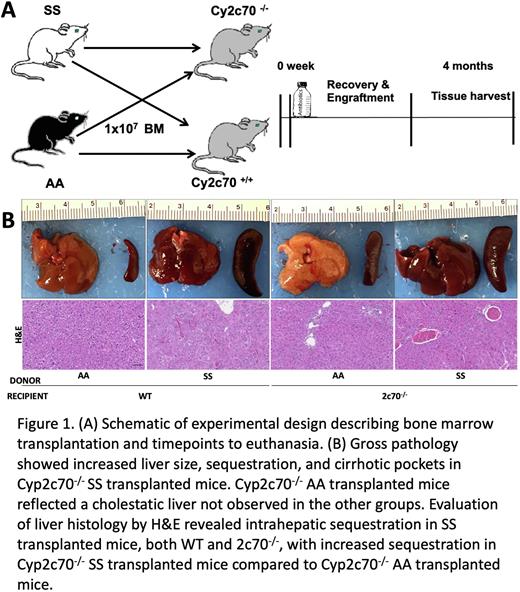
Methods:Tibias and femurs were excised from 2-month-old SCD SS and AA control mice and the bone marrow (BM) was collected. After lethal irradiation (2x 4.5Gy 4 hours apart), 2-month-old male recipient Cyp2c70-/- and their wildtype controls received BM-derived cells from either AA or SS mice. Cyp2c70-/- (KO) mice (C57Bl/6) were generated using CRISPR/Cas9 technology. Recipient Cyp2c70-/- and WTmice were reconstituted with SS and AA bone marrow and were euthanized 4 months after BMT. Standard growth parameters were recorded; liver, spleen, gallbladder bile, and blood were collected at necropsy for histologic, RNA, and chemical analyses.
Results: Recipient Cyp2c70-/- (KO) SS mice had a significantly increased liver-to-body weight ratio compared to their controls. There was an increase in serum bile acids, direct bilirubin, and total bilirubin in SS recipient Cyp2c70-/- and WT mice compared to AA recipient mice. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were increased in Cyp2c70-/- (KO) overall with no observable difference in Cyp2c70-/- (KO) transplanted with AA or SS. An evaluation of liver histology by H&E revealed increased portal bridging and hepatic sequestration in sinusoids of recipient Cyp2c70-/- SS mice compared to control mice. Liver surface nodularity and morphological heterogeneity were also present in recipient Cyp2c70-/- SS mice compared to their controls. We observed increased collagen deposition as measured by Sirius Red staining in recipient Cyp2c70-/- SS mice compared to control mice. Liver histology revealed increased hepatic inflammation by F4/80 staining and bile duct proliferation by CK-19 and CK-8 staining in recipient Cyp2c70-/- SS mice.
Conclusion: Coupled with the humanized sickle cell disease mouse, this novel sickle cell cholestatic liver mouse model can be used to investigate the role of hydrophobic bile acid-induced injury in sickle cell-related hepatobiliary disease and screen new therapeutic approaches for SCH.
Disclosures
Dawson:Albireo Pharma: Research Funding. Karpen:Albireo: Consultancy; Mirum: Consultancy; Vertex: Consultancy; Intercept: Consultancy. Archer:Forma Therapeutics: Research Funding; Global Blood Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal